From Solid
to Plasma
Solids and Liquids
When a solid changes into a liquid it is called melting very similar to how ice melts into water. When a liquid changes into a solid it is called freezing same with how water changes into ice. One thing of note is that when something goes through any phase change it will not change temperatures until it is done changing phases.

Liquids and Gases
When liquids turn into a gas it is called evaporation or boiling. Boiling is what water does at 212F or 100C. Evaporating is what any liquid does over time at a much to release energy and lower its temperature. When gases turn into a liquid it is called condensing. An example of this is when you have a cold drink on a hot day and the liquid cools down the surrounding water vapors.

Solids and Gases
When Solids turn directly into a gas skipping the liquid phase it is called Sublimination. This is why CO2 does. In fact liquid CO2 is impossible under the regular Earth atmosphere. When a gas turns into a solid it is called Deposition. This is also possible in nature but almost never happens at room temperatures in fact you often have to freeze CO2 in order to see this effect.

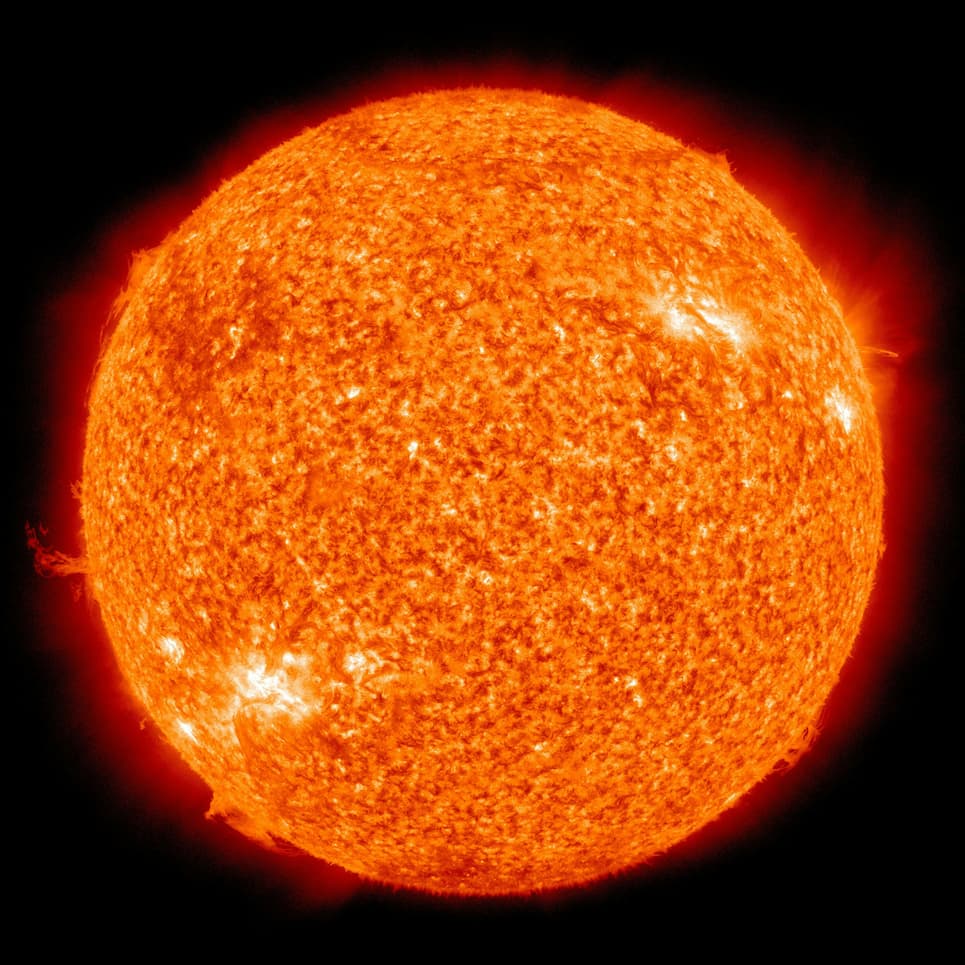
Plasma
Anything can turn into Plasma it just must reach the temperature of around 5000K in order to convert into this change. This is rarely something that is seen outside of lightning, the sun, or in special vacuums made to host gases that are electrically charged.